LSPedia 的全球序列化系列
序列化不是一刀切的。由于美国、欧盟、亚洲和中东的规定各不相同,公司必须应对复杂的要求网络。您为全球合规做好准备了吗?
.avif)
欢迎来到 LspEdia,在这里,创新与奉献精神相结合。
如果你热衷于有所作为并在协作环境中茁壮成长,LspEdia 就是你的不二之选。

序列化不是一刀切的。由于美国、欧盟、亚洲和中东的规定各不相同,公司必须应对复杂的要求网络。您为全球合规做好准备了吗?
.avif)
如果你热衷于有所作为并在协作环境中茁壮成长,LspEdia 就是你的不二之选。


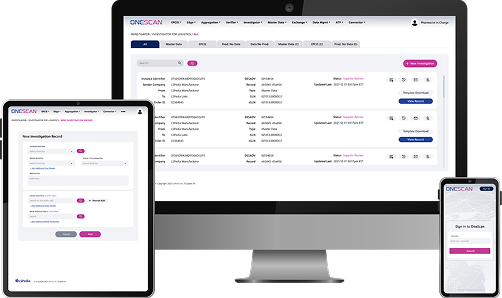
Enhanced Suspect Order Monitoring (SOM) is a OneScan module built for manufacturers, distributors, and other regulated stakeholders in the pharmaceutical supply chain to identify and manage potentially suspicious orders for controlled substances.
.webp)

Simplifies identification, tracking, and evaluation of suspicious ordering activity.

Supports compliance with DEA requirements to detect orders of unusual size, frequency, or deviation from normal patterns.

Reduces time, labor, and manual review effort by consolidating monitoring and reporting into a single system.

Improves visibility into customer ordering trends across large customer networks and high order volumes.

Enable consistent, defensible compliance decisions and help demonstrate due diligence during regulatory audits.
The Enhanced SOM module delivers scalable, cloud-based suspicious order monitoring that improves visibility, consistency, and efficiency across high-volume customer networks. As part of the OneScan solution suite, Enhanced SOM simplifies compliance while minimizing impact on daily operations.
Automatically evaluate controlled-substance orders as they are placed.
Aggregate ordering behavior at the active ingredient (molecule) level and identify irregular volume across products and strengths.
Use historical ordering data to establish baselines and configure time windows and thresholds based on SOPs or best practices.
Filter suspicious activity by customer, molecule, or NDC and drill down to detailed order and product information.
Set variance percentages and limits aligned to internal policies and identify statistical outliers within customer ordering behavior.
Integrate with ATP for license verification and leverage FDA NDC and reference data within OneScan.
Export flagged orders and reports to CSV, XLSX, PDF, and other formats.
Define sensitive ingredient grouping and automatically monitor orders involving those groupings.